Dr. Jontana Allkja, Postdoctoral research assistant Oral Sciences Research group, Dental School and Hospital, University of Glasgow. In collaboration with Prof. Gordon Ramage, Infection Prevention & Control, Safeguarding Health through Infection Prevention Research Group, Research Centre for Health, School of Health and Life Sciences, Glasgow Caledonian University.
Wound infections represent a significant problem within the healthcare system, making up the majority of healthcare associated infections, killing an estimated 37,000 people per year within the EU alone, and leading to costs of €7 billion per year for the sector. An example of these wounds are diabetic foot ulcers (DFUs), a grave complication of diabetes. There are over 4.5 million diabetics in the UK, with a 34% lifetime risk of developing a DFU. Around 50% of these DFUs become infected and among these 85% have an associated biofilm (a community of bacterial and fungal cells surround by a self-produced matrix structure). Unfortunately, this leads to a difficulty to treat them as biofilms are tolerant to most conventional treatments, moreover the presence of various bacterial and fungal species coexisting and cooperating within it and the rise of antimicrobial resistance make it even more difficult to treat them. This means we need to look for new approaches to treat these infections.
I am part of a collaborative project between the University of Glasgow, Glasgow Caledonian University, the University of Bath and Lancaster University. In our team, we have chemists, engineers and materials specialists who are developing wound dressings containing antimicrobial compounds, as well as a device to generate cold atmospheric plasma (CAP) (Fig 1).
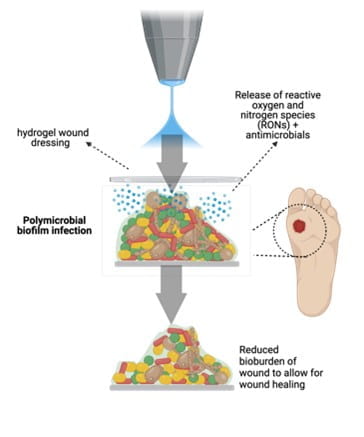
The latter will be used to release the drug from the gel, as well as promote healing due to its effect on the cells. I am one of the microbiologists in this team, who is tasked with testing these therapies to ensure they are safe and effective.
I developed many skills relevant to this work during my PhD at the University of Porto, Portugal, in collaboration with Montana State University, USA. I was part of collaborative European training Network (ETN) project called Print-aid, involving collaborators from Finland, Belgium, the Netherlands, Italy and Poland. We aimed to develop 3D printed implants with anti-infective capabilities. During this time, I had the opportunity to interact with many engineers and biomaterials developers and learn about the process of developing and testing these devices. Moreover, my project focused on the development of reliable and reproducible biofilm testing methods, as such I gained a lot of experience in developing protocols and even validating them as standard methods, which government agencies like the FDA use as testing methods before putting products in the market. I had the opportunity to perform a multi-laboratory trial, involving 6 labs across Europe and the US, to test the protocols I developed for different biofilm testing methods. I also worked on developing methods to assess multispecies biofilms of urinary catheters using different techniques, including microscopy. I will use this expertise to develop complex biofilm models, attempting to mimic the wound environment in the lab, that we can then use to test the dressings and CAP to ensure they are effective.
To find out more about the SHIP team, head on to the GCU website, read the rest of our blogs and follow us on Twitter @SHIPGCU
